Why Biofilms Make Diabetic Foot Ulcers Hard to Treat
Touro Researchers Describe the Role of Biofilms in Foot Ulcers and Novel Anti-Biofilm Strategies to Speed Recovery
Foot ulcers in people with diabetes are a growing problem across the world, with an estimated 26 million new cases annually. Yet, treatments often fail, and the ulcers often lead to serious complications and even amputation. Treatments fail largely because the wounds are “protected” by biofilms that make them less penetrable by antibiotics.
Researchers at Touro’s New York College of Podiatric Medicine (NYCPM) and the Touro College of Osteopathic Medicine recently published a review of biofilms in diabetic foot ulcers (DFUs), current treatment protocols, and novel therapies that may more effectively treat them. The review was published in the journal Acta Microbiologica Hellenica.
“We took on this research because biofilms are under-recognized but central to diabetic foot ulcer development and to treatment failure,” says Paramita Basu, PhD, MS, associate professor and director of Infectious Diseases at NYCPM.
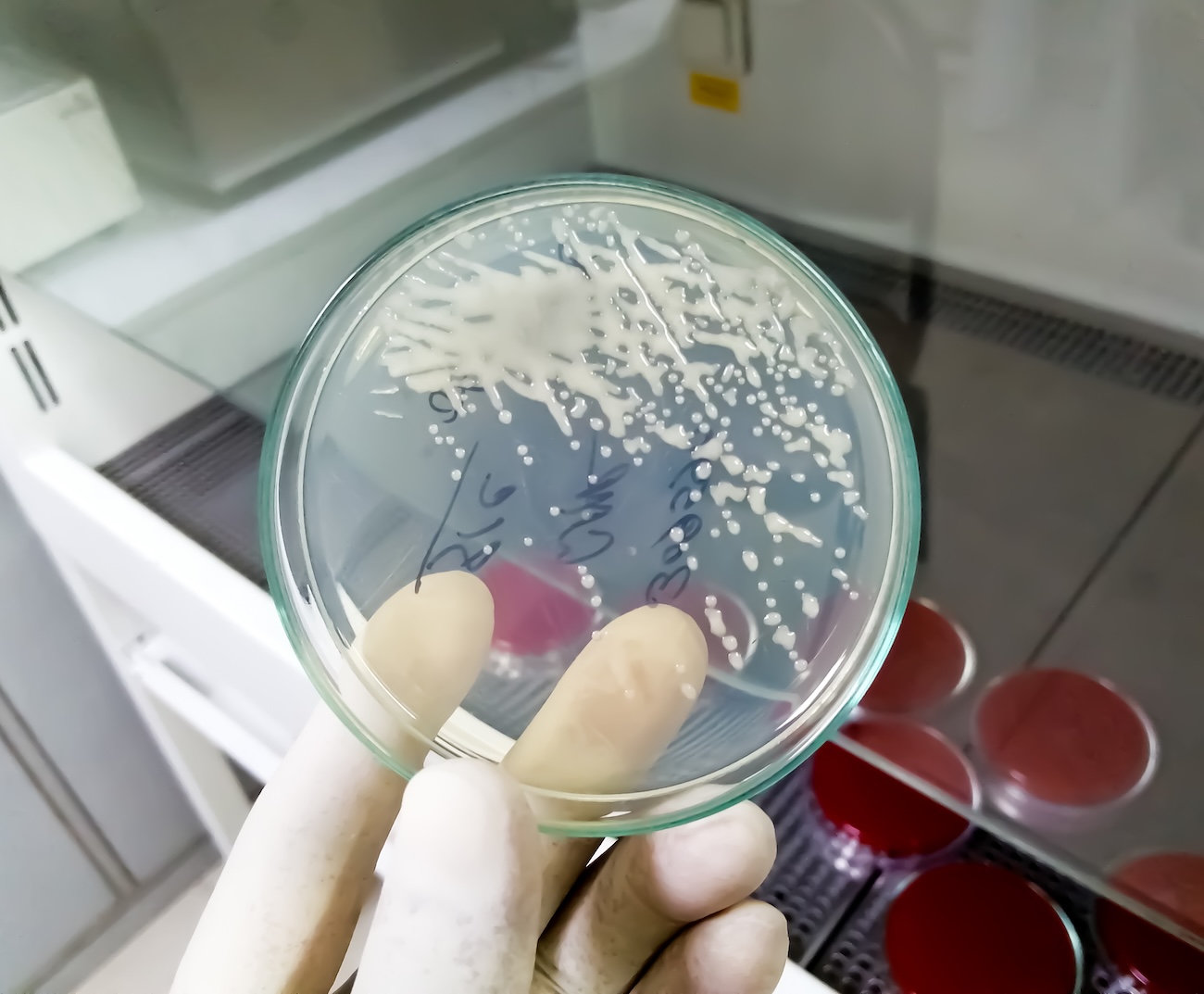
“Biofilms are slimy layers made by bacteria that stick to wounds, surfaces, or devices,” explains Dr. Basu. “They’re like a protective layered ‘house’ that bacteria build using sugars and proteins.”
People with diabetes often have high blood sugar levels, which creates an ideal environment for the growth of bacteria, specifically Pseudomonas aeruginosa and Staphylococcus aureus. These bacteria produce toxins and other substances that form biofilms, which help them hide from antibiotics and the immune system.
“They’re very common in chronic infections, especially in wounds that don’t heal well, like diabetic foot ulcers,” says Dr. Basu. Almost every diabetic foot ulcer (DFU) has a biofilm.
The comprehensive review describes the mechanisms of biofilm formation, and how they create such an antibiotic-resistant armor. Based on a literature review, they found that a tougher biofilm was associated with more severe DFU, poorer outcomes, and greater risk of amputation.
Understanding these mechanisms and the bacteria that are involved in biofilms can help guide antibiotic treatment as well as the development of new targeted treatments, says Dr. Basu. For instance, most biofilms have multiple bacteria that need to be targeted with multiple antibiotics.
They also reviewed the effectiveness of other commonly used therapies, like debridement, in which dying or dead tissue around the wound is removed using a scalpel or a jet spray of water.
In addition to current treatments, they also described novel therapies that attack the biofilm, such as antibiotic peptides, the use of placental-derived materials and human umbilical cord grafts, photodynamic therapy, nanomaterials, phage therapy (using viruses that infect and kill bacteria), and other innovative approaches.
The authors concluded that the main goal in treating chronic DFUs is to target the biofilms, as their formation significantly delays wound healing. This typically requires a multidisciplinary approach, that includes strict adherence to appropriate antibiotics, often with surgical removal of dying tissue, and the use of additional novel approaches.
“We hope to encourage research and clinical adoption of newer anti-biofilm strategies,” says Dr. Basu, who stressed the importance of targeted, pathogen-specific therapies.
As for preventing DFUs, the authors emphasized that blood sugar control is key to prevention and to healing. Likewise, early attention to foot injuries may prevent ulcers from escalating.